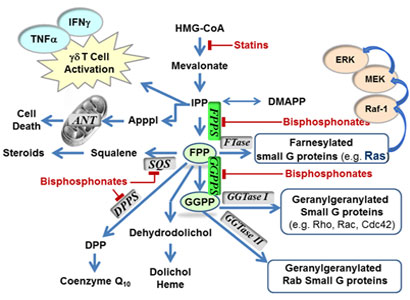
Human farnesyl pyrophosphate synthase (hFPPS) is responsible for the biosynthesis of farnesyl pyrophosphate (FPP) and controls intracellular levels of all down-stream isoprenoids, including geranylgeranyl pyrophosphate (GGPP). FPP and GGPP are essential for the post-translational prenylation of small GTPases, a family of signaling proteins that are fundamentally important for cell survival. Farnesylated proteins (e.g. H-Ras, K-Ras and N-Ras) are predominant in oncogenesis. Nitrogen-containing bisphosphonates (N-BPs) are the only clinically validated drugs that target hFPPS. They are potent inhibitors of osteoclastic activity and are widely used for the treatment of bone-related diseases. Recent clinical investigations provided evidence that N-BPs are also disease modifying agents that improve survival in patients with breast cancer, prostate cancer and multiple myeloma via mechanisms unrelated to their skeletal effects. In addition to down-regulating Ras prenylation, inhibition of hFPPS also induces apoptosis stimulates the human innate immune response against tumors and pathogens.
- "Human farnesyl pyrophosphate synthase (hFPPS)"
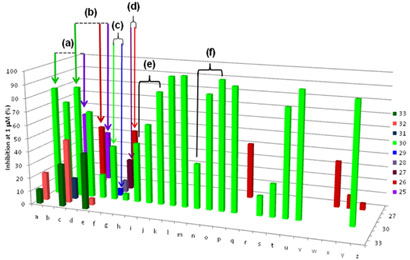
- "High throughput in vitro screening of compound libraries"
One of our goals is to design novel inhibitors of hFPPS that exhibit good distribution in non-skeletal tissues as pre-clinical candidates for the development of therapeutics for the treatment of cancer. Structural modifications that probe the size, shape, electrostatic surface complimentarily and binding mode of our inhibitors with the active site of hFPPS are in progress. Our studies include the evaluation of both N-BP and non-N-BP molecules in enzymatic assays and cell-based assays using human multiple myeloma cells.