Our Research Focuses on Engineering Phosphorus-Containing Molecules that are useful as (I) Leads for Medicinal Chemistry and/or (II) Tools for Asymmetric Synthesis.
There are obvious benefits to combining studies in novel synthetic methodologies with studies in medicinal chemistry. Under the large umbrella of our two research themes (I and II), several of our projects represent unique examples of the extensive “cross-pollination” between our efforts in medicinal chemistry (I) and those in catalytic and asymmetric synthesis (II).
Some Recent Examples from Medicinal Chemistry and Chemical Biology:
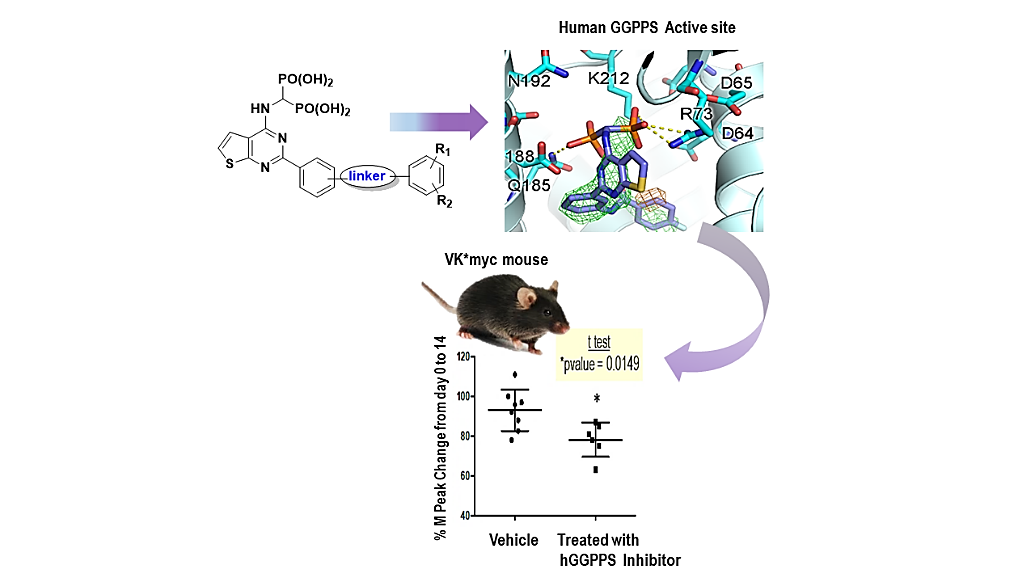
1. Lacbay et al. Unraveling the Prenylation-Cancer Paradox in Multiple Myeloma with Novel Geranylgeranyl Pyrophosphate Synthase (GGPPS) Inhibitors. J. Med. Chem. 2018, 61, 6904-6917.
In collaboration with Prof. Albert Berghuis (Professor and Chair Biochemistry Department, McGill University) and Dr. Michael Sebag (MD/PhD; Department of Medicine, Division of Hematology McGill University Health Center):
We have identified a new class of thienopyrimidine-based bisphosphonate (ThP-BP) inhibitors of the human geranylgeranyl pyrophosphate synthase (hGGPPS) that block protein prenylation in multiple myeloma (MM), a blood cancer which is currently incurable. These inhibitors block the proliferation of cancer cells, leading to apoptosis. Administration of one inhibitor a MM mouse model, confirmed in vivo downregulation of Rap1A geranylgeranylation and reduction of monoclonal immunoglobulins, which are a biomarker of disease burden in the serum. These results provide the first proof-of-principle that hGGPPS is a valuable therapeutic target for the treatment of multiple myeloma.
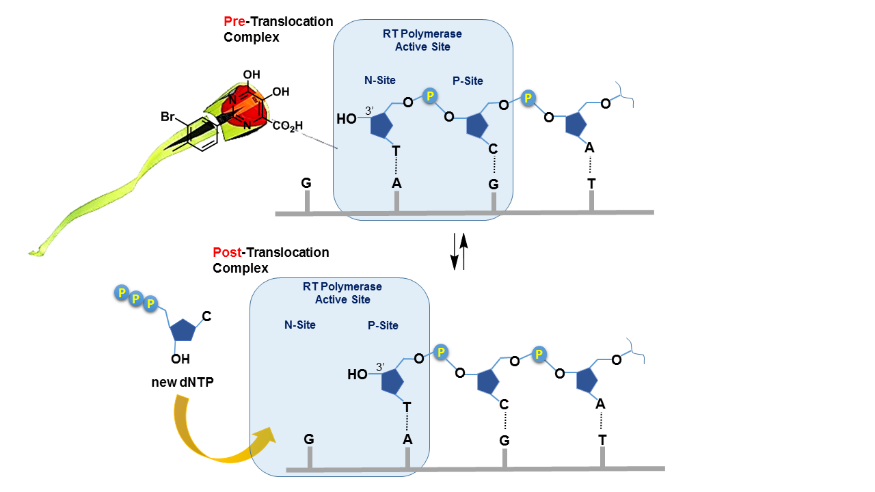
2. Lacbay et al. Pharmacophore Requirements for HIV-1 Reverse Transcriptase Inhibitors that Selectively “Freeze” the Pre-Translocation Complex During the Polymerization Catalytic Cycle. Bioorg. Med. Chem. 2018, 26, 1713-1726.
In collaboration with Prof. Matthias Götte (Professor and Chair, Department of Medical Microbiology and Immunology; University of Alberta):
Reverse transcriptase (RT) is responsible for replicating the HIV-1 genome and is a validated therapeutic target for the treatment of HIV infections. During each cycle of the RT-catalyzed DNA polymerization process, inorganic pyrophosphate is released as the by-product of nucleotide incorporation. Inhibitors were identified that act as bioisosteres of pyrophosphate and can selectively freeze the catalytic cycle of HIV-1 RT at the pre-translocated stage of the DNA- or RNA-template-primer-enzyme complex.
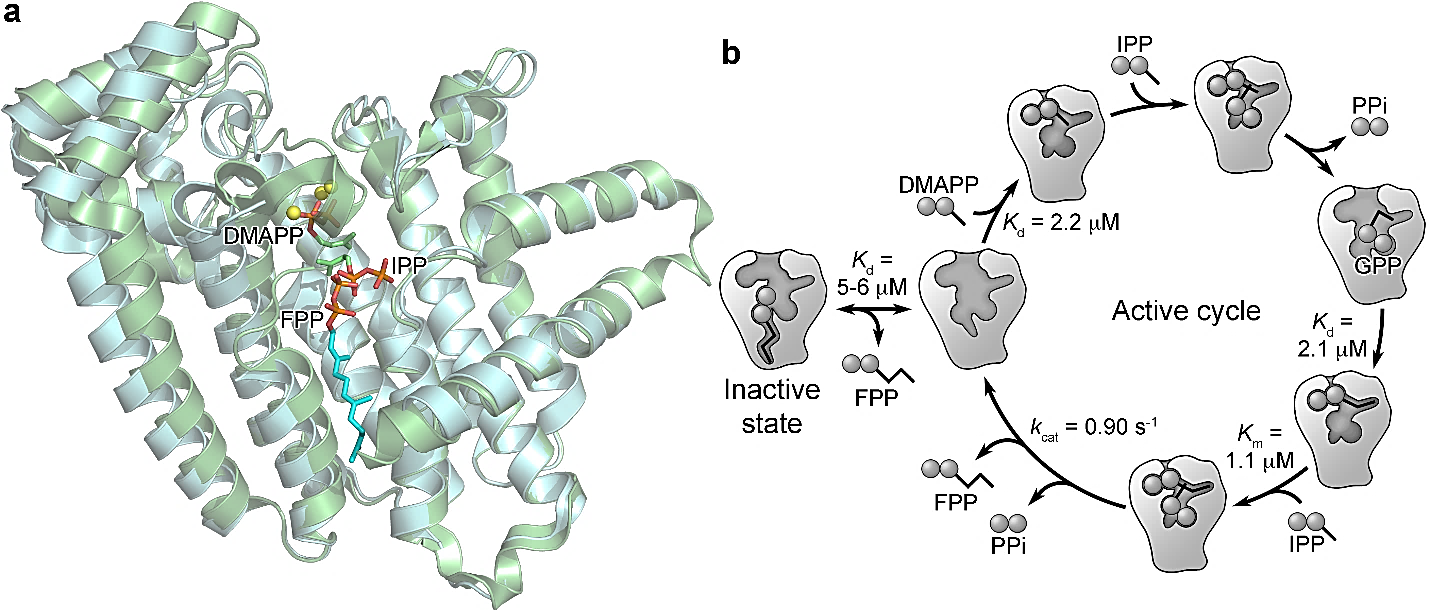
3. (a) Park et al. Pharmacophore Mapping of Thienopyrimidine-Based Monophosphonate (ThP-MP) Inhibitors of the Human Farnesyl Pyrophosphate Synthase
J. Med. Chem. 2017, 60, 2119-2134.
(b) Park et al. Human farnesyl pyrophosphate synthase is allosterically inhibited by its own product. Nature Commun. 2017, 8, 14132. Selected for Nature Commun website and recommended in F1000Prime for special significance in the field, in collaboration with Prof. Albert Berghuis:
Human FPPS plays a key role in the prenylation of small GTPases, which are intimately involved in oncogenesis. An allosteric pocket of the enzyme has been of particular interest as a therapeutic target. However, its natural biological function has been (until now) unknown. We identified that the catalytic product of hFPPS, farnesyl pyrophosphate (FPP), bind to this pocket and locks the enzyme in a conformationally inactive state. Therefore, this allosteric binding site offers an exquisite mechanism for controlling the intracellular levels of isoprenoid biosynthesis in vivo.
Some Recent Examples from Synthesis and Catalysis:
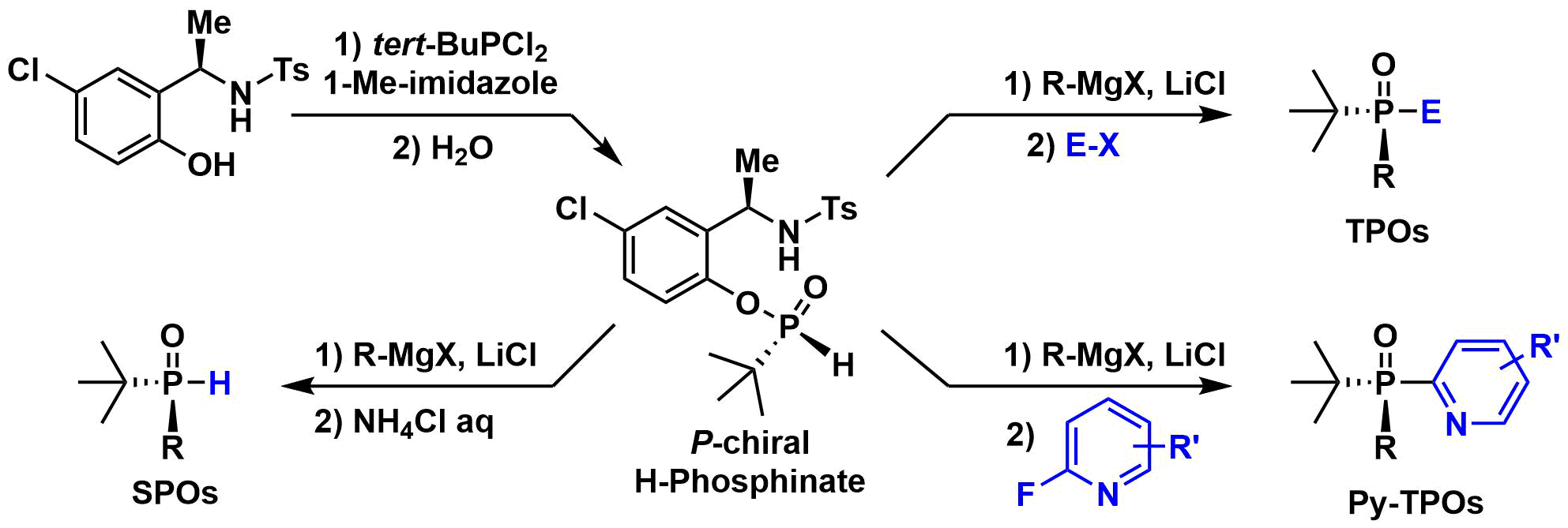
1. Li, S. et al. Asymmetric Library Synthesis of P-Chiral t-Butyl-Substituted Secondary and Tertiary Phosphine Oxides. J. Org. Chem. 2019, 84, 7291-7302.
An asymmetric synthesis, amenable to library preparation of structurally diverse P-chiral t-butyl substituted secondary phosphine oxides (SPOs) and tertiary phosphine oxides (TPOs) was developed. A P-chiral H-phosphinate building block was prepared via a 2-step, one pot condensation of a chiral auxiliary with t-BuPCl2, followed by hydrolysis. Nucleophilic displacement of the chiral auxiliary with Grignard reagents, followed by hydrolysis provided a library of P-chiral SPOs. In situ treatment of the pre-hydrolysis intermediate with electrophiles also provided a library of P-chiral TPOs in high enantiomeric purity.
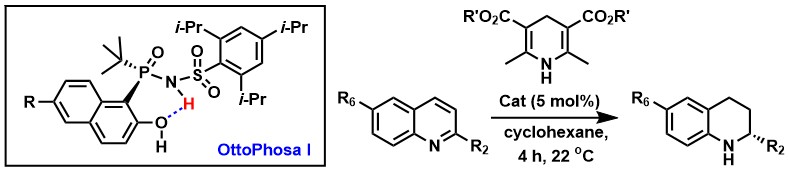
2. Yuan, M. et al. P-Chiral, N-phosphoryl sulfonamide Brønsted acids with an intramolecular hydrogen bond interaction that modulates organocatalysis Org. Biomol. Chem. 2019, 17, 8690-8694.
Brønsted acids exemplified by OttoPhosa I were designed and evaluated in the asymmetric transfer hydrogenation of quinolines. Their catalytic properties are modulated by an intramolecular hydrogen bond that rigidifies their catalytic cavity, accelerates the reaction rate and improves enantioselectivity.
Our research is multidisciplinary, involving a seamless integration of fundamental and applied sciences, and provides an excellent training ground for students and Post-Doctoral Fellows interested in pursuing an academic or industrial career.